Research
Analysis of Sex Differences in Cardiometabolism and Gene Regulation
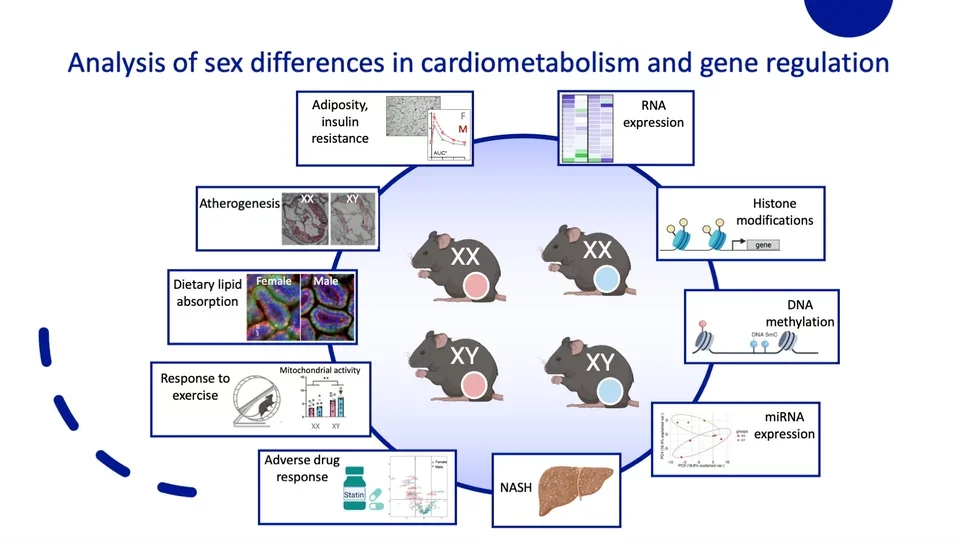
Our lab aims to understand sex differences in cardiometabolism and gene regulation. The Four Core Genotypes mouse model (pictured) can be utilized to identify aspects independently regulated by sex chromosomes or gonadal hormones. We have evaluated the contribution of these sex components on atherogenesis, adiposity, adverse drug response, microRNA expression, and RNA expression. Our ongoing research projects aim to delineate the impact of each sex component on dietary lipid absorption, response to exercise, nonalcoholic steatohepatitis (NASH), and gene regulation through epigenetic modifications including histone marks and DNA methylation.
Sex differences in obesity
Men and women become fat in different ways. Hormones produced by male and female gonads are a key factor, but not the only factor. Our studies in mice have shown that the presence of XX chromosomes promotes obesity compared to XY chromosomes when studied in XX female, XX male, XY female and XY male mice. We have identified specific X chromosome genes that are likely to contribute and are characterizing their effects using knockout and transgenic mouse models.
Brown adipose tissue and energy metabolism
In addition to a role for fat tissue in energy storage, brown adipose tissue is specially designed to expend energy through heat generation. Mechanisms that increase brown fat thermogenesis have potential utility in reducing obesity. In studies performed in mice and humans, we have identified genes and small molecule compounds that are associated with a propensity for increased energy dissipation. Ongoing studies aim to characterize the mechanisms by which they influence thermogenesis.
Sex differences in cardiometabolic disease
Cardiovascular disease, specifically atherosclerosis, differs between the sexes in regard to plaque type, cellular composition, and clinical presentation. We have identified that mice with two X chromosomes (XX) have an increase in atherosclerosis compared to mice with one X chromosome (XY). Our ongoing studies will use single-cell RNA sequencing to further delineate the effect of gonadal and chromosomal sex on atherosclerotic plaque development. In addition, the role of specific X and Y chromosome genes on atherosclerosis will be investigated using knockout and transgenic mouse models.
Pharmacogenomics of statin drugs
Statin drugs are among the most widely prescribed drugs in the world, and are effective for reduction of blood cholesterol levels and cardiovascular disease. Some individuals taking statins experience adverse effects, notably myopathy (muscle damage) and new onset diabetes. We are utilizing a genetic screen in mice to identify the genes and pathways that influence these adverse responses to statin drugs.
Sex differences in postprandial lipid metabolism and inflammation
Consumption of a fat-rich meal causes an increase in circulating triglyceride levels and inflammatory response. Biological sex has been identified as a factor influencing postprandial hyperlipidemia and inflammation. We have identified a role for gonadal sex impacting postprandial hyperlipidemia, while the chromosomal sex influences the postprandial inflammatory response. Ongoing studies aim to delineate the mechanisms contributing to the sex-dependent regulation of postprandial lipid metabolism and inflammation.
Lipin proteins—masters of fat synthesis
Many years ago, we used a mutant mouse strain that cannot store fat tissue to identify the lipin gene family. We have shown that lipins regulate fat synthesis and storage in adipose tissue, muscle, liver, intestine and other sites. Using knockout mouse strains and CRISPR-mutated cell lines, we are characterizing roles for lipin proteins in lipid synthesis, autophagy, inflammation, cell differentiation, and regulation of gene expression.